
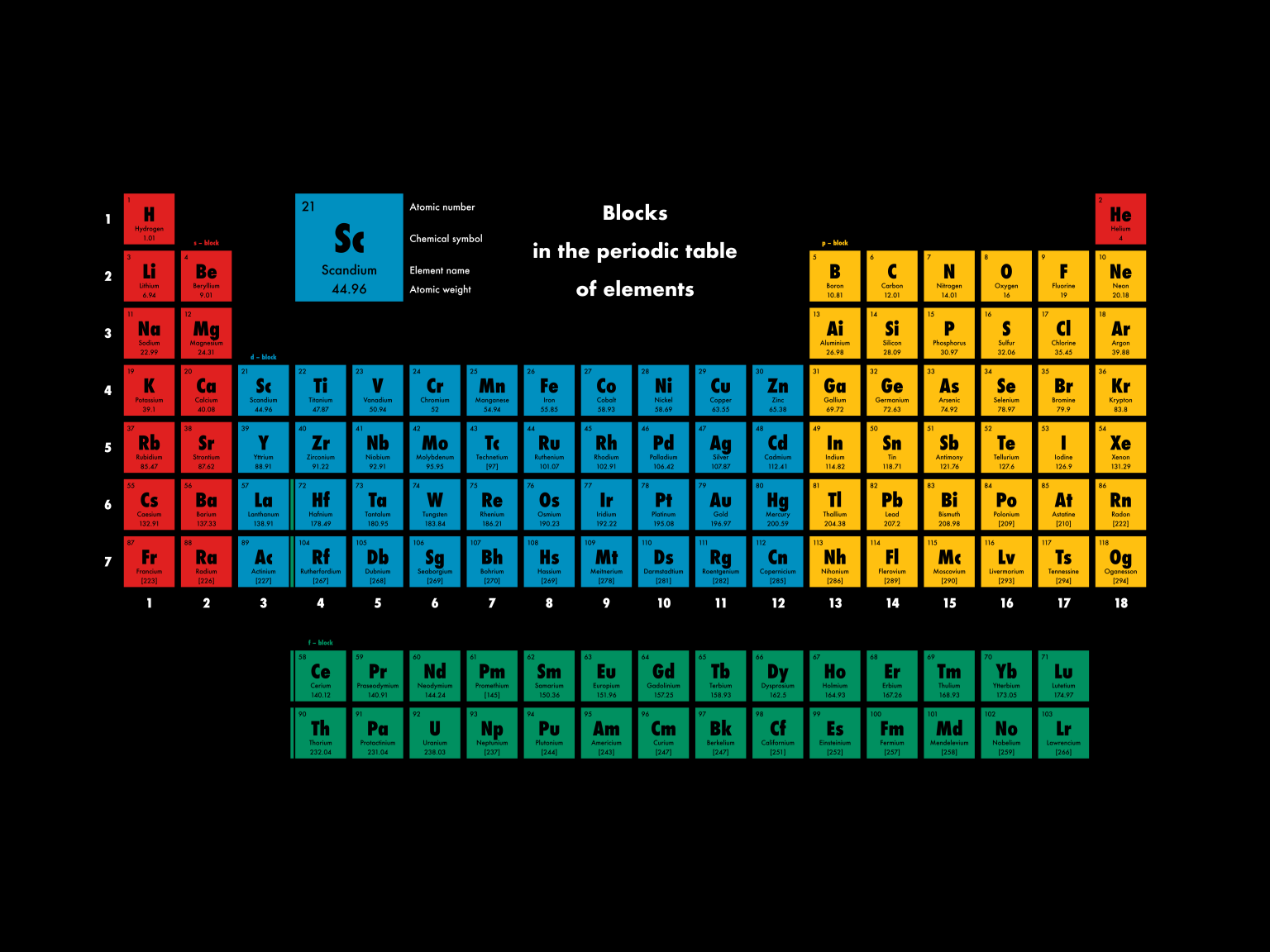
“Similar to electrons, when nuclear orbits are filled with protons, they form stable nuclei, analogous to the noble-gas elements,” says collaborator Kouichi Hagino. The Nucletouch table places these ‘magic nuclei’ at its center, providing a new perspective on the elements. Among these are familiar elements such as helium, oxygen, and calcium. Protons have different stable magic numbers: 2, 8, 20, 28, and so on. Imagining that protons in a nucleus exist in ‘orbits’ may seem like a stretch, but the discovery of the concept was awarded the 1963 Nobel prize in physics. Maeno describes these as atomic ‘magic numbers’, and importantly the same principle can also be applied to protons. Their most stable electron numbers are 2, 10, 18, 36, and so on.” “So-called ‘noble gases’, inert elements such as helium, neon, and argon, rarely react with other elements. Atoms are considered to be stable when electrons completely fill their ‘shell’ of orbits around the nucleus,” continues Maeno.

“Fundamentally, it comes down to the electrons in each atom. He even had the foresight to add space for elements that were still unknown in his time. Over 150 years have passed since Dmitri Mendeleev discovered the periodic law that lead him to propose the classic periodic table. Credit: Kyoto University/Yoshiteru Maeno/Kouichi Hagino The fundamental elements organized by their proton ‘magic number’.


 0 kommentar(er)
0 kommentar(er)
